BASAGLAR KWIKPEN
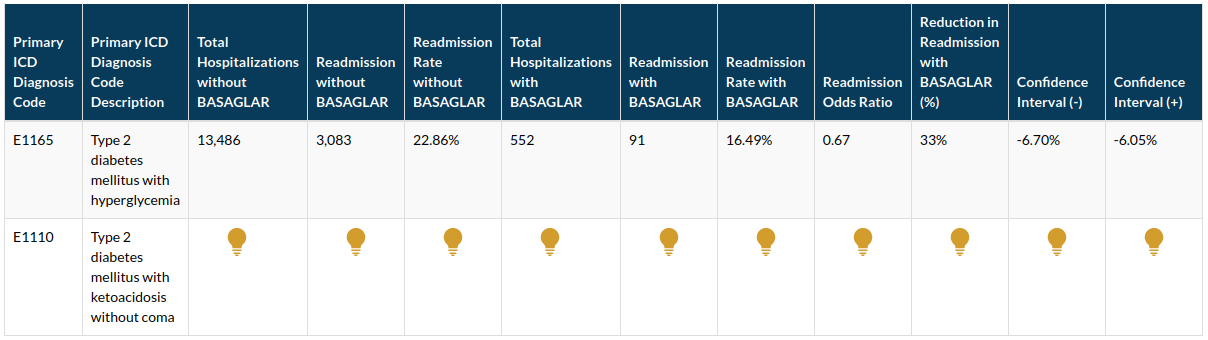
Type II Diabetes is frequently associated with hospital readmission. Readmission is a hospital quality measure that reflects dimensions of quality of inpatient care and also impacts overall programs by payers such as CMS’s Star Rating Program, HRRP and Value-Based Purchasing. BASAGLAR KWIKPEN U-100(insulin glargine) is a disposable, single-patient-use prefilled insulin pen. BASAGLAR was the first insulin product approved through an abbreviated approval pathway by the FDA. Dexur’s analysis of Real-World Evidence based on Medicare Claims data showed that the use of BASAGLAR KWIKPEN U-100(insulin glargine) within 7 days of hospitalization was associated with lower hospital readmission rates for Type II Diabetes. Reduction in readmission rates helps hospitals improve their outcomes for various cost and quality programs. Dexur is an approved entity to perform Medicare claims data analysis, which it uses to perform quality outcomes analysis. Dexur has published several papers with Harvard Medical School on quality outcomes related topics. Dexur’s analysis of Drugs and their impact on quality outcomes, such as readmissions and mortality, are used by Hospitals, IDNs, and ACOs to implement best practices and manage Real World Evidence based risk factors.
Read more