Xolair patient share was 1.6x more than Nucala and 2x compared to Fasenra in Asthma treatment in 2019
Get Dexur’s Personalized Hospital Specific Presentation on Quality, Safety, Compliance & Education
By: Sruthy Iype Sep. 21, 2020
Dexur’s analysis of Medicare claims data showed that Xolair’s patient share for asthma was 1.6 times more than Nucala and twice that of Fasenra in 2019. The study was based on the J code usage of biologic therapies - Xolair, Nucala, Fasenra, and Cinqair - for asthma among Medicare patients who received treatments in hospital outpatient settings between Jan and Dec 2019. The analysis also looks at the average claims per patient data, which is a proxy for the number of injections / IV infusions / doses administered to a patient.
Achieving disease control, measured by the frequency and severity of exacerbations (asthma attacks) and lung function, is a major challenge especially among patients suffering from severe asthma. Advancements in the use of biologics, as an add on to other asthma treatments including corticosteroids, has proved to be beneficial in tackling this aspect of disease management. Existing biologic therapies for asthma mainly target the IgE (Immunoglobulin E) and IL (Interleukin) -5/4/13 mediated pathways responsible for airway inflammation. Among the drugs considered in this study, Genentech’s Xolair (omalizumab) is an anti-IgE antibody, GlaxoSmithKline’s Nucala (mepolizumab) and Teva’s Cinqair (reslizumab) are IL-5 antagonists, while AstraZeneca’s Fasenra (benralizumab) is an IL-5 receptor antagonist.
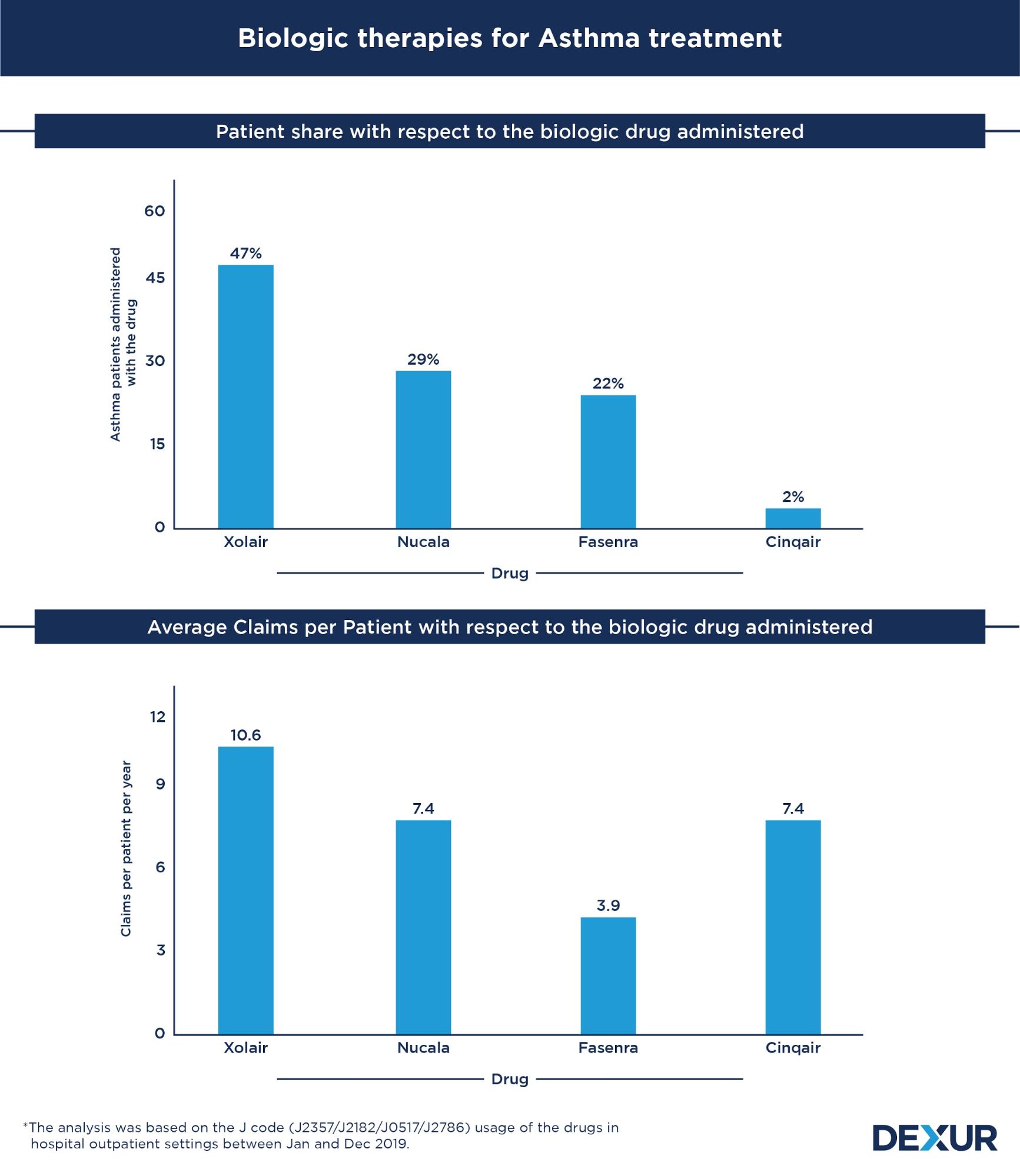
The largest proportion of asthma patients were treated with Xolair among the four biologics considered under this study. The first biologic drug for asthma to hit the market, Xolair’s patient share (47 percent) exceeded that of its competitors’ by a huge margin. It also recorded the highest average usage of 10.6 claims per patient in a year. Its closest competitor, Nucala, had a patient share of 29 percent, with an average of 7.4 claims per patient. Close on its heels is Fasenra, which had a patient share of 22 percent.
The last among the four to be launched in the market, Fasenra has managed to capture a significant share of the market in a short time. Having an average of 3.9 claims per patient, the drug has the advantage of having a lower dosage frequency compared to its competitors. While Fasenra is usually administered to asthma patients once in 8 weeks, its competitors - Xolaris, Nucala, and Cinqair - have dosage frequencies ranging between 2 to 4 weeks.
With a significantly smaller patient proportion, Cinqair had a share of 2 percent of the total asthma patients considered under the study. Similar to the usage pattern observed for Nucala, patients had an average of 7.4 claims each for the drug in a year.