More than 50% of Keytruda’s usage is associated with Lung Cancer Treatment
Get Dexur’s Personalized Hospital Specific Presentation on Quality, Safety, Compliance & Education
By: Sruthy Iype Aug. 26, 2020
Dexur’s analysis of Medicare claims data showed that lung cancer patients accounted for more than 50 percent of Keytruda’s usage, followed by patients of melanoma, bladder cancer, head & neck cancer, and kidney cancer. The analysis looked at the J code (J9271) usage of the drug among Medicare patients* who received outpatient care between Jan and Dec 2019. The analysis also looks at the average claims per patient data, which is a proxy for the number of injections / IV infusions / doses administered to a patient.
Keytruda (pembrolizumab) is an immunotherapy drug by Merck used in the treatment of various advanced types of cancer, either as a monotherapy or in combination with other drugs. It belongs to the class of drugs called PD-1 (Programmed cell death protein 1) inhibitors. By targeting PD-1, an inhibitory receptor protein that plays an important role in the regulation of T-cell activity, the drug restores the ability of the immune system to detect and destroy the cancer cells. The FDA approved indications of Keytruda include Melanoma, Non-Small Cell Lung Cancer, Small Cell Lung Cancer, Renal Cell Carcinoma, Classical Hodgkin Lymphoma, Endometrial Carcinoma, and Squamous Cell Carcinoma of the Head and Neck. This study examines the use of Keytruda with respect to the top 5 types of cancer where its usage has been observed.
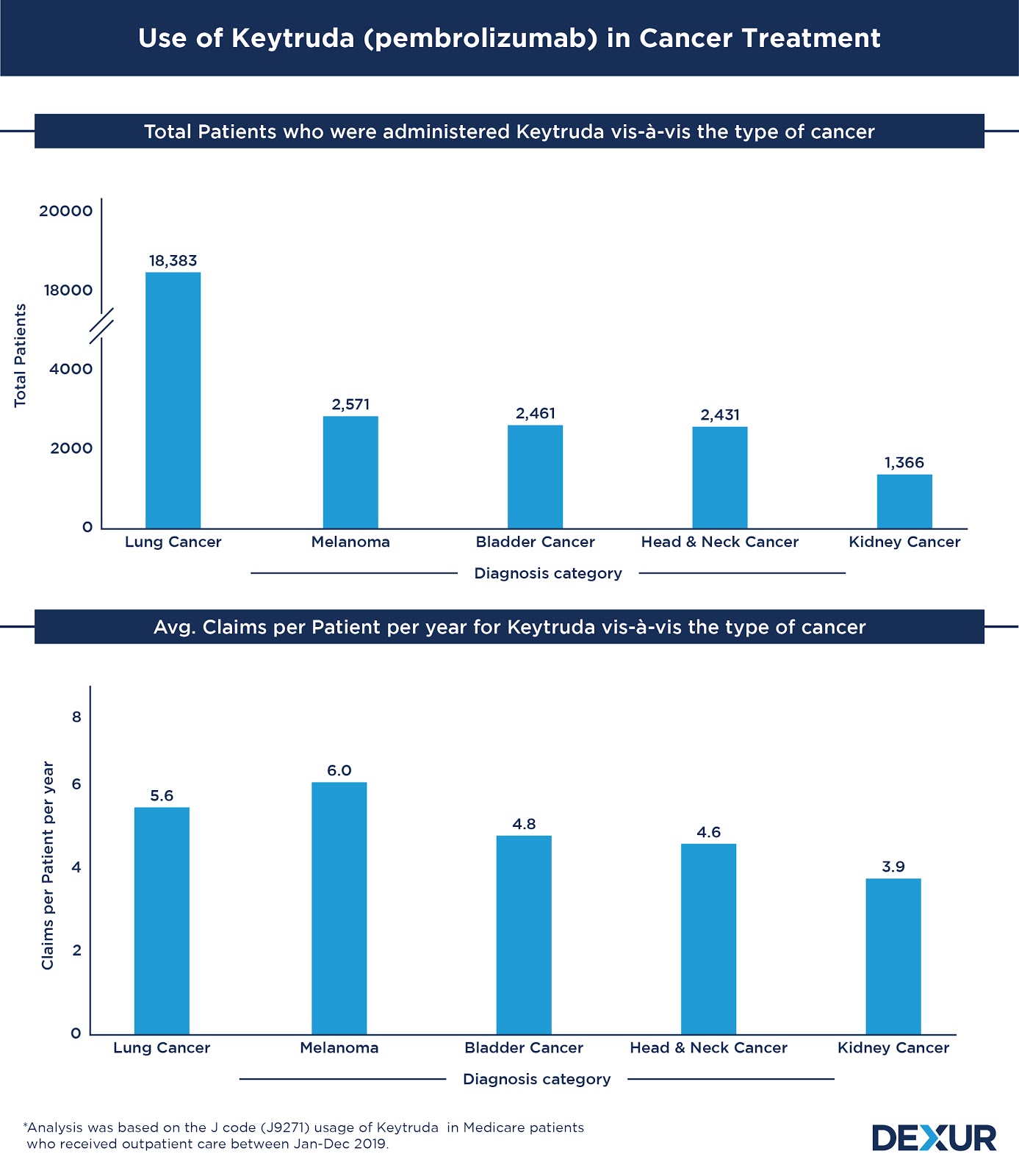
| Diagnosis Category | Total Patients | Claims per Patient per year |
|---|---|---|
| Lung Cancer | 18,383 | 5.6 |
| Melanoma | 2,571 | 6 |
| Bladder Cancer | 2,461 | 4.8 |
| Head & Neck Cancer | 2,431 | 4.6 |
| Kidney Cancer | 1,366 | 3.9 |
More than 50 percent of Keytruda’s usage can be attributed to the treatment of lung cancer. The drug, approved for both Non-Small Cell Lung Cancer and Small Cell Lung Cancer, was administered to a total of 18,383 patients belonging to the diagnosis category between Jan and Dec 2019. Lung Cancer patients had an average of 5.6 claims per year for the drug.
With a total of 2,571 patients, Melanoma forms the second largest diagnosis category associated with the use of Keytruda. It had a relatively higher average usage per patient of 6.0 claims per year. Following melanoma, the use of Keytruda was observed to be prevalent in the treatment of bladder cancer and head & neck cancer. The drug was administered to 2,461 patients of bladder cancer and 2,431 patients of head & neck cancer during this period. While the patients belonging to the former diagnosis category had an average of 4.8 claims, those falling under the latter had 4.6 claims each.
Significant use of the drug was also seen in association with the treatment of kidney cancer. During the period of study, a total of 1,366 patients of kidney cancer were given Keytruda as a part of their treatment, wherein each patient had an average of 3.9 claims each.
* Individuals who received treatment for more than one type of cancer were considered as unique patients/claims under each of the concerned diagnosis categories, for the purpose of this analysis.