Head and neck cancer accounts for nearly 50 percent of the total patients administered Erbitux
Get Dexur’s Personalized Hospital Specific Presentation on Quality, Safety, Compliance & Education
By: Sruthy Iype Sep. 04, 2020
Dexur’s analysis of Medicare claims data showed that head and neck cancer accounts for nearly 50 percent of the total patients who were administered Erbitux, followed by colorectal cancer and squamous cell carcinoma of the skin. The analysis was based on the J code (J9055) usage of the drug among Medicare patients who received outpatient care between Jan 2017 and Dec 2019.
Erbitux (cetuximab) is a monoclonal antibody that belongs to the drug class called epidermal growth factor receptor (EGFR) inhibitors. The binding of epidermal growth factor to EGFR, a transmembrane protein found on the surface of cells, sets off a series of events that result in cell growth and differentiation. In certain types of cancer, the overexpression of EGFR was found to be responsible for unregulated cell proliferation and tumor growth. By targeting the receptor and blocking the events mediated by it, Erbitux helps in slowing down the progression and spread of the disease. It is approved as a targeted therapy, monotherapy or in combination with other drugs, in the treatment of certain types of colorectal, and head and neck cancer.
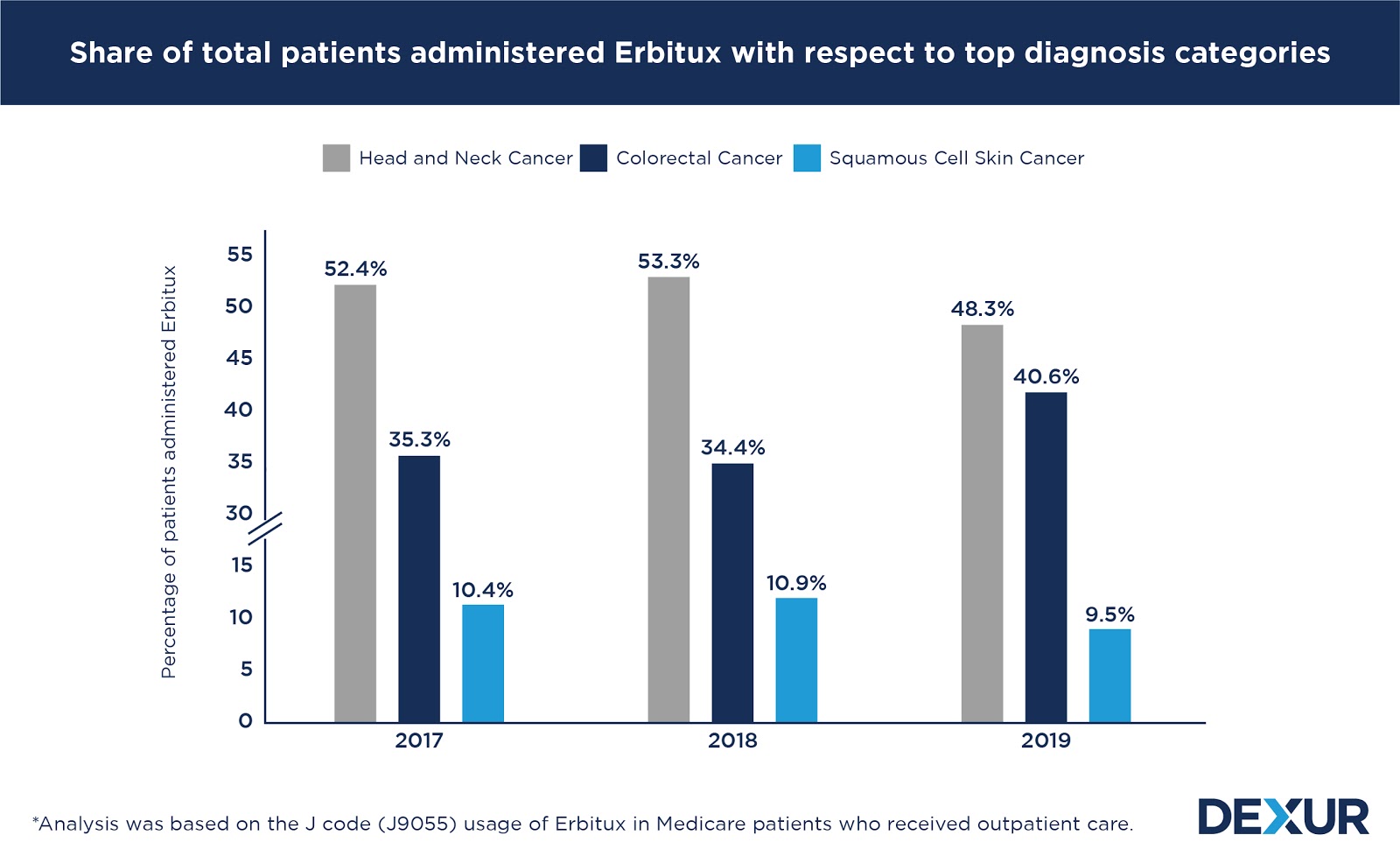
Head and neck cancer patients account for the largest share of Erbitux’s usage, with nearly half of the total patients who were administered the drug belonging to the diagnosis category. After maintaining a share of about 53 percent consecutively in 2017 and 2018, the diagnosis category’s share of total patients diminished to 48 percent in 2019.
Following head and neck cancer, the use of the drug was noted to be most prevalent in the treatment of colorectal cancer. Contrary to the trend observed in the case of the former, the diagnosis category’s share of the total patients who were administered Erbitux saw a significant growth from an average of 35 percent in 2017 and 2018, to 41 percent in 2019.
A considerable proportion of the drug’s usage was also associated with the treatment of squamous cell carcinoma of the skin. The diagnosis category’s share of the total patients who received Erbitux was between 10 to 11 percent during the three years considered in the study.