Newly Approved Giapreza Highlights Importance of Hospital Septic Shock Related Mortality Reduction
Get Dexur’s Personalized Hospital Specific Presentation on Quality, Safety, Compliance & Education
By: Saparja Nag Feb. 12, 2018
The FDA recently approved Giapreza (angiotensin II), by La Jolla Pharmaceuticals, an injection for intravenous infusion to increase blood pressure in adults with septic or other distributive shock. In the FDA statement, Norman Stockbridge, M.D., Ph.D., director of the Division of Cardiovascular and Renal Products in the FDA’s Center for Drug Evaluation and Research, said "Shock, the inability to maintain blood flow to vital tissues, can result in organ failure and death. There is a need for treatment options for critically ill hypotensive patients who do not adequately respond to available therapies."
Sepsis is one of the largest causes of inpatient hospitalizations. Naturally, hospital physicians, pharmacists, and healthcare quality professionals want to understand how this newly approved drug could potentially improve outcomes for patients with septic shock. Dexur has developed several hospital specific dashboards and analyses for key therapeutic areas (see this example collaboration with Harvard) that hospitals can use to monitor & improve outcomes. Dexur’s sepsis and septic shock dashboard based on Medicare and other claims databases helps hospital quality teams understand mortality rates, length of stay (LOS), readmission rates and other quality outcomes by hospital for septic shock-related hospitalizations. The data from these dashboards are also regularly published to help the larger patient community make the appropriate healthcare choices.
The below chart shows mortality rates for inpatients with septic shock at the national level compared to selected teaching hospitals based on Medicare claims data from January 2013 to December 2016. The teaching hospitals included in this analysis were Yale-New Haven Hospital, Brigham and Women’s Hospital, and Duke University Hospital. These statistics are not adjusted for DRG level risk and other patient factors such as age.
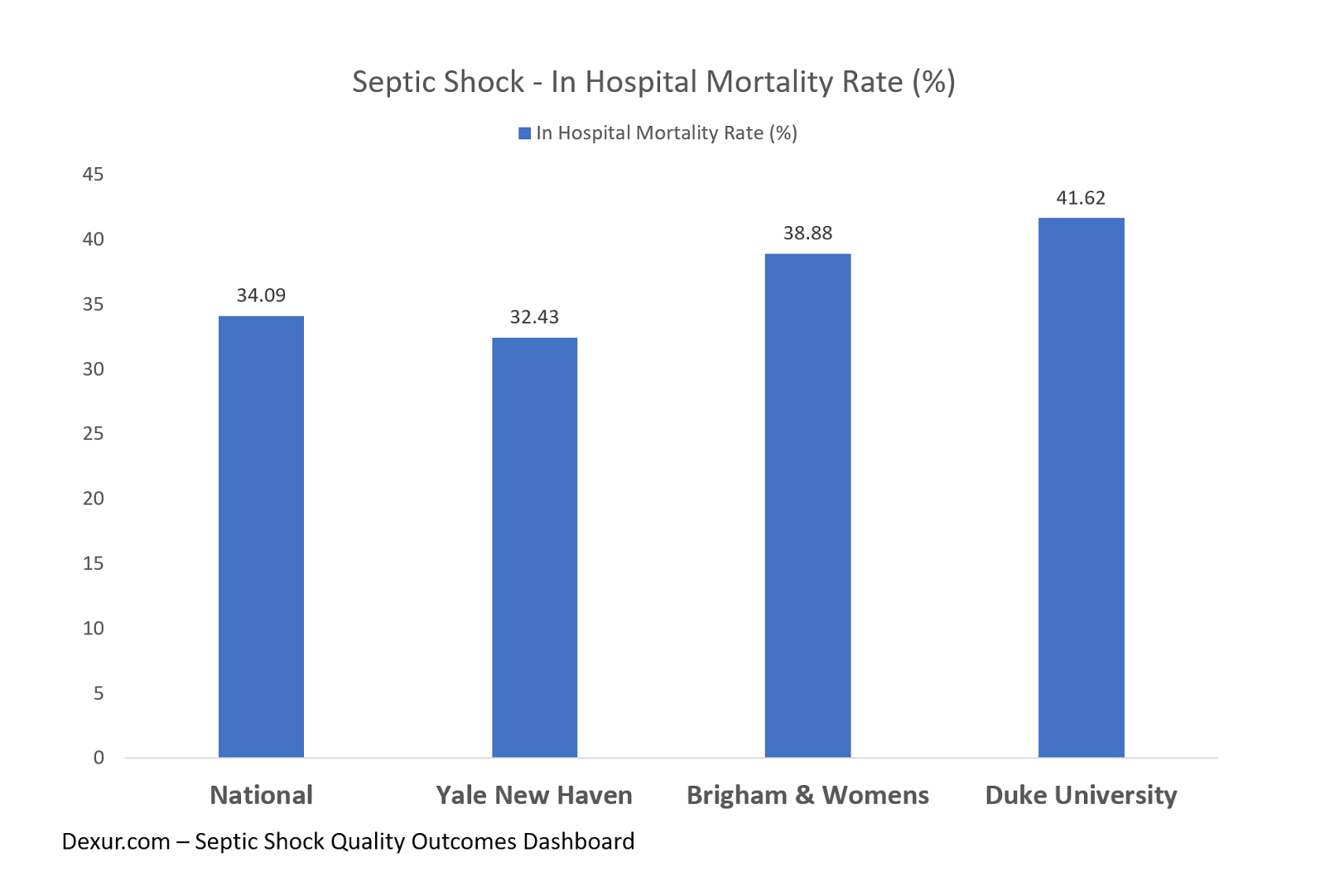
The data confirms the FDA’s assessment of the association between septic shock and a high mortality rate within the hospital setting, and thus the need for a drug like Giapreza. Analysis in future articles on Giapreza will include coverage on septic shock and hypotensive comorbidities, as well as readmission rates and LOS.
DEXUR PRO MEMBERS GET ACCESS TO:
- Mortality rates for septic shock at the top 10 Hospitals by Medicare hospitalization volume
- Mortality rates for the same top 10 hospitals when septic shock occurs while admitted in the hospital
ABOUT THE AUTHOR
