Cimzia is used more for Rheumatoid Arthritis compared to Psoriatic Arthritis, Ankylosing Spondylitis, and Crohn’s Disease
Get Dexur’s Personalized Hospital Specific Presentation on Quality, Safety, Compliance & Education
By: Sruthy Iype Sep. 02, 2020
Dexur’s analysis of Medicare claims data showed that Cimzia was used more in the treatment of rheumatoid arthritis, as compared to psoriatic arthritis, ankylosing spondylitis, and Crohn’s disease. The analysis was based on the J code (J0717) usage of the drug among Medicare patients who received outpatient care between Jan and Dec 2019. The analysis also looks at the average claims per patient data, which is a proxy for the number of injections / IV infusions / doses administered to a patient.
UCB’s Cimzia (certolizumab pegol) is a monoclonal antibody used in the treatment of inflammatory and autoimmune disorders including rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and plaque psoriasis. It is a biologic that belongs to the drug class called tumor necrosis factor (TNF) blocker. The excessive production of TNF-alpha, a signalling protein that plays a key role in regulating immunological responses, contributes to the pathogenesis of autoimmune disorders. By neutralizing the protein, Cimzia helps to manage the symptoms of the disease including inflammation and pain. This study examines the top diagnosis categories where the use of the drug has been observed.
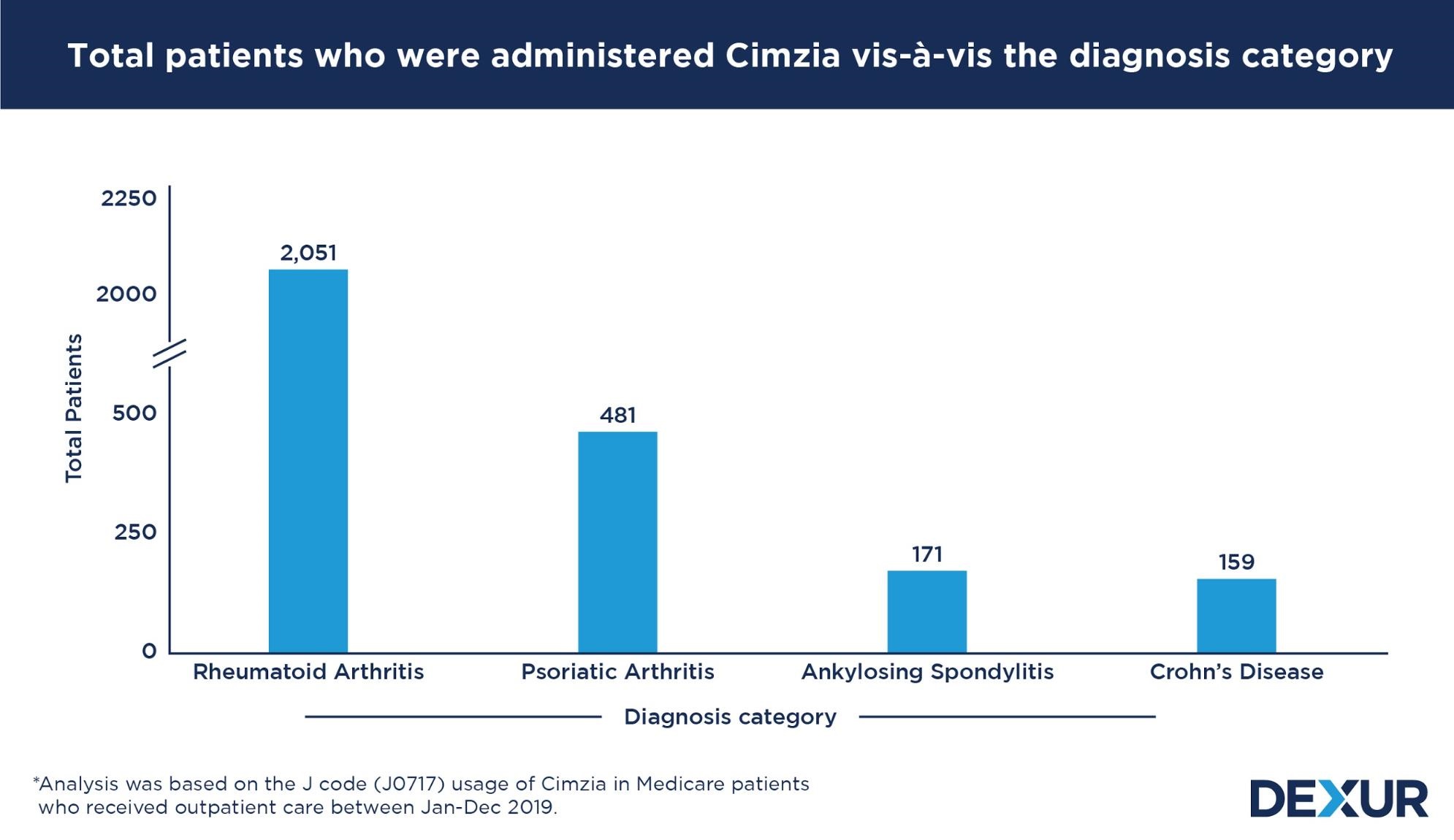
| Diagnosis Category | Total Patients | Claims per Patient per year |
|---|---|---|
| Rheumatoid Arthritis | 2,051 | 7.6 |
| Psoriatic Arthritis | 481 | 7.1 |
| Ankylosing Spondylitis | 171 | 7.0 |
| Crohn’s disease | 159 | 8.6 |
The use of Cimzia in the treatment of rheumatoid arthritis, accounts for more than half of the drug’s total usage. Between Jan and Dec 2019, the drug was administered to a total of 2,051 patients belonging to this diagnosis category, wherein each patient had an average of 7.6 claims per year.
Following rheumatoid arthritis, the use of the drug was most prevalent in the treatment of psoriatic arthritis. The 481 patients belonging to this category, had an average of 7.1 claims for the drug during this period.
Considerable use of the drug was also observed in the treatment of ankylosing spondylitis and Crohn’s disease. During the period of study, the drug was administered to 171 patients of ankylosing spondylitis and 159 patients of Crohn’s disease as a part of their treatment. While patients of the former category had an average of 7.0 claims per year, those belonging to the latter had a relatively higher average usage of 8.6 claims per year.